Explain why a single carbonyl band would be expected in the system and why this vibration is located at 1673 cm-1 . – Essaylink
Get your Assignment in a Minimum of 3 hours
Our academic experts are ready and waiting to assist with any writing project you may have. From simple essay plans, through to full dissertations, you can guarantee we have a service perfectly matched to your needs.
Free Inquiry1.Conjugation of the functional group in alkyl isocyanates has little impact on the antisymmetric ![]() stretching vibration located near 2770 cm–1 Explain.
stretching vibration located near 2770 cm–1 Explain.
2.In the infrared spectrum of 2-aminoanthraquinone (I) two carbonyl stretching frequencies are observed at 1673.5 and 1625 cm–1 :
(a) Assign carbonyl bands in the infrared spectrum to the carbonyl groups in structure I and explain your reasoning
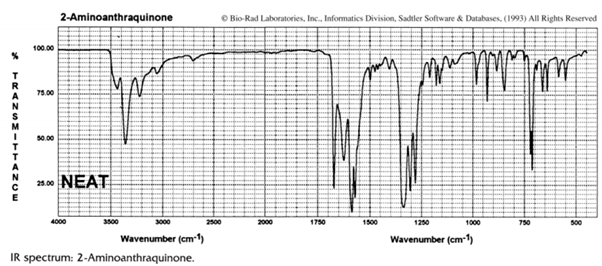
(b) The infrared spectrum of 1-hydroxyanthraquinone (II) also exhibits two carbonyl frequencies, which are located at 1675 and 1637 cm–1 . Assign the carbonyl groups to the related absorption bands. Explain your reasoning.
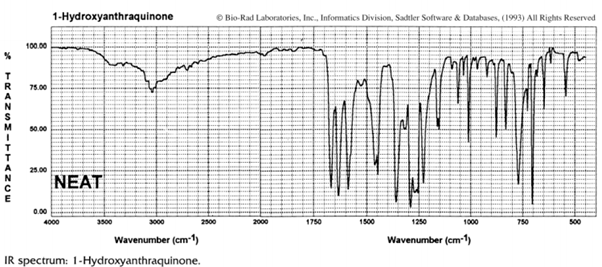
(c) The spectrum of 2-hydroxyanthraquinone exhibits a single carbonyl stretching frequency near 1673 cm–1 . Explain why a single carbonyl band would be expected in the system and why this vibration is located at 1673 cm–1 .

The post Explain why a single carbonyl band would be expected in the system and why this vibration is located at 1673 cm-1 . appeared first on Best Custom Essay Writing Services | EssayBureau.com.
"Is this question part of your assignment? We Can Help!"
"Our Prices Start at $11.99. As Our First Client, Use Coupon Code GET15 to claim 15% Discount This Month!!"
Get Started
